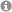UR Home
UR Home
Unnatural Amino Acids and Peptids
Unique α,β- and α,α,β,β-Peptide Foldamers based on cis-β-Aminocyclopentanecarboxylic Acid
L. Berlicki, L. Pilsl, E. Weber, I. M. Mandity, C. Cabrele, T. A. Martinek, F. Fülöp, O. Reiser, Angew. Chem. 2012, 124, 2251-2255; Angew. Chem. Int. Ed. Engl. 2012, 51, 2208-2212
Web edition: http://dx.doi.org/10.1002/anie.201107702
Foldamers stable in water: cis-β-Aminocylopentanecarboxylic acid proves to be a unique building block for the synthesis of α,β- and α,α,β,β-peptides displaying unique helical structures withhigh stability in methanol and aqueous media.
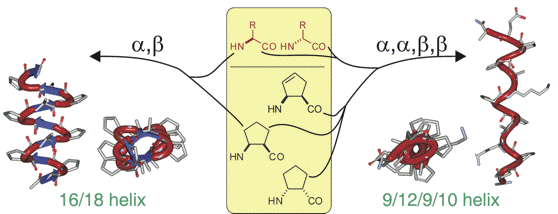
Synthesis of (S)- and (R)-5-Oxo-piperazine-2-Carboxylic Acid and Its Application to Peptidomimetics.
K. Guitot, S. Carboni, O. Reiser, U. Piarulli , J. Org. Chem. 2009, 74, 8433-8436
Web edition: http://dx.doi.org/10.1021/jo901389q
A straightforward synthesis of (S)- and (R)-N-Boc-5-oxo-piperazine-2-carboxylic acid, is reported starting from L- or D-serine and ethyl glyoxylate. Those were evaluated as constituents in two tetrapeptides by studying their secondary structure by 1H-NMR spectroscopy. In the case of Boc-Val-(S)-PCA-Gly-Leu-OMe, two readily interconverting conformations (in a 40:60 % ratio) were observed, differing for the cis-trans isomerizaton of the tertiary amide bond, while, Boc-Val-(R)-PCA-Gly-Leu-OMe, displayed an equilibrium between a γ-turn and a type II β-turn conformation.
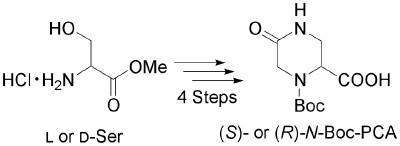
The Constrained Amino Acid β-Acc Confers Potency and Selectivity to Integrin Ligands
S. Urman, K. Gaus, Y. Yang, U. Strijowski, N. Sewald, S. De Pol, O. Reiser, Angew. Chem. 2007, 119, 4050-4053; Angew. Chem. Int. Ed. 2007, 46, 3976-3978
Web edition: http://dx.doi.org/10.1002/anie.200605248
Complete opposites: The incorporation of the rigid building block (+)-b-Acc (Acc=aminocyclopropane carboxylic acid) into cyclic RGD peptides results in a high affinity towards the integrin avb3. The peptides 1 and 2 are composed of the enantiomeric building blocks (+)- (blue) and (-)-β-Acc (red). The active peptide 1 inhibits integrin avb3 mediated cell adhesion to vitronectin with an IC50 value of 20 nm.
Identification of the Key Residue of Calcitonin Gene Related Peptide (CGRP) 27-37 to Obtain Antagonists with Picomolar Affinity at the CGRP Receptor
M. Lang,
S. De Pol,
C. Baldauf,
H.-J. Hofmann,
O. Reiser,
A. G. Beck-Sickinger, J. Med. Chem. 2006, 49, 616-624
Web edition: http://dx.doi.org/10.1021/jm050613s
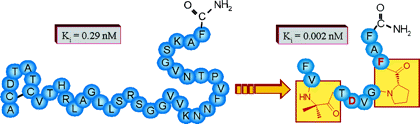
Calcitonin gene related peptide (CGRP) plays an important role in the CNS and in the cardiovascular system. To identify high-affinity antagonists in competitive binding studies, we identified a novel radioactive tracer, [3H-propionyl-K24]-hRCGRP 8-37, which was labeled in solution by a recently developed strategy using photolabile protecting groups at reactive side chains. This tracer was shown to be as potent as commercially available 125I-tracers for the determination of agonists and to have increased sensitivity for antagonists. We applied it to investigate the predicted turn structures centered at Pro29 and Pro34. The substitution at positions 29 and 34 by turn-inducing amino acid mimetica showed that these turns are highly diverse. At position 29, a hydrophobic residue is preferred that constricts the secondary structure, whereas position 34 is required to stabilize the conformation of the backbone. All high-affinity analogues showed antagonistic properties with potency similar to CGRP 8-37.
Structural properties of orexins for activation of their receptors
M. Lang, B. Bufe, S. De Pol, O. Reiser, W. Meyerhof, A. Beck-Sickinger, J. Pept. Sci. 2006, 12, 258-266
Web edition: http://dx.doi.org/10.1002/psc.716
The closely related neuropeptides orexin A and orexin B mediate their actions, including the regulation of sleep and appetite, by the activation of the orexin 1 and 2 receptors. To elucidate the structural prerequisites for receptor activation and subtype selectivity, we performed multiple amino acid substitutions within the sequence of orexin A and human orexin B-(6-28)-peptide and analyzed their solution structures by CD spectroscopy and their activity at both receptors in Ca2+ mobilization assays. For orexin A, we showed that the basic amino acids within the segment of residues 6–14 were important for the activation of both receptors. Furthermore, we showed that the restriction via disulfide bonds is not required to maintain the active structure of orexin A. The kink region of h orexin B has been shown to be important for Ox2R selectivity, which is not mediated by the restriction of the turn structure. Additionally, we showed that no particular secondary structure is required for receptor subtype selectivity.
Stereoselective synthesis of novel conformationally restricted β- and γ-amino acids
F. Gnad, M. Polschak, O. Reiser, Tetrahedron Lett. 2004, 45, 4277-4280
Web edition: http://dx.doi.org/10.1016/j.tetlet.2004.04.013
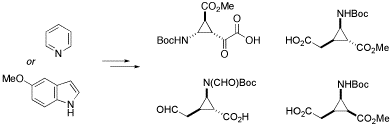
Novel conformationally restricted β- and γ -amino acids containing a cyclopropane ring could be stereoselectively synthesized from readily available 5-methoxyindole and pyridine by copper(I)-catalyzed cyclopropanation with methyl diazaoacetate followed by subsequent oxidative cleavage of the resulting adducts.
Surprisingly Stable Helical Conformations in α/β-Peptides by Incorporation of Cis-β-Aminocyclopropane Carboxylic Acids
S. de Pol, C. Zorn, O. Zerbe, O. Reiser, Angew. Chem. 2004, 116, 517-520; Angew. Chem. Int. Ed. Engl. 2004, 43, 511-514
Web edition: http://dx.doi.org/10.1002/anie.200352267
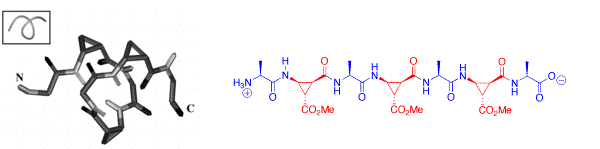
The alternating combination of (L)-alanine and of cis-β-aminocyclopropanecarboxylic acids (cis-β-ACCs) results in acyclic α/β-peptides that form stable 313 helix turns in solution with as little as seven residues. The chirality of the cis-β-ACCs was found to be crucial for the formation of this new class of foldamers.
Analogues of Neuropeptide Y Containing β-Aminocyclopropane-carboxylic Acids are the Shortest Linear Peptides Selective for the Y1-Receptor
N. Koglin, C. Zorn, R. Beumer, C. Cabrele, C. Bubert, N. Sewald, O. Reiser, A. G. Beck-Sickinger, Angew. Chem. 2003, 115, 212-215; Angew. Chem. Int. Ed. Engl. 2003, 42, 202-205
Web edition: http://dx.doi.org/10.1002/anie.200390078
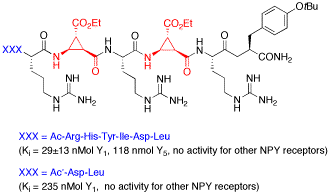
For the first time truncated linear peptides containing a unique conformationally restricted β-amino acid were obtained, which show high and selective affinity toward the Y1 receptor of Neuropeptide Y. High affinity of these ligands was dependant on the position and the configuration of this β-aminocyclopropane-carboxylic acid.
Deprotection of N-alloc amines by Pd(0) /DABCO: an efficient method for in situ peptide coupling of labile amino acids
C. Zorn, F. Gnad, S. Salmen, T. Herpin, O. Reiser, Tetrahedron Lett. 2001, 42, 7049-7053
Web edition: http://dx.doi.org/10.1016/S0040-4039(01)01453-8
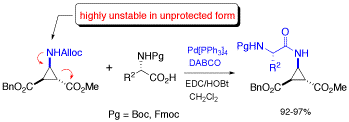
A highly efficient one-pot deprotection/peptide coupling protocol of N-Alloc amino acids with activated N-Boc or N-Fmoc amino acid was developed in solution and on solid phase. DABCO was found to be especially effective for the deprotection of the N-Alloc group, resulting in short reaction times (10-20 min) and allowing the coupling of amino acids that are not stable in unprotected form.
β-Aminocyclopropanecarboxylic Acids with α-Amino Acid Side Chain Functionality
R. Beumer, O. Reiser, Tetrahedron 2001, 6497-6503
Web edition: http://dx.doi.org/10.1016/S0040-4020(01)00541-5
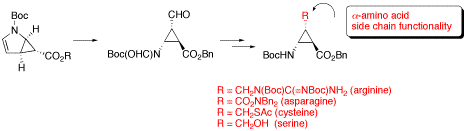
The synthesis of conformationally restricted trans-β-aminocyclopropanecarboxylic acids (β-ACCs) having α-amino acid side chain functionality of asparagine, arginine, cysteine, or serine is described.
The Synthesis of Diastereo- and Enantiomerically Pure β-Aminocyclopropane Carboxylic Acids.
R. Beumer, C. Bubert, C. Cabrele, O. Vielhauer, M. Pietzsch, O. Reiser, J. Org. Chem. 2000, 65, 8960-8969
Web edition: http://dx.doi.org/10.102/jo005541l
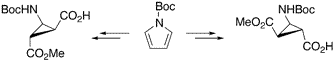
The synthesis of diastereo- and enantiomerically pure β-aminocyclopropanecarboxylic acids (β-ACCs) is described. Both, cis or trans β-ACCs, being conformationally restricted β-alanine or γ-aminobutyric acid (GABA) derivatives, represent useful building blocks for peptides containing unnatural amino acids.