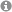UR Home
UR Home
Catalysis
Visible Light Photoredox Catalysis: Generation and Addition of N-Aryltetrahydroisoquinoline Derived α-Amine Radicals to Michael Acceptors
P. Kohls, D. Yadav, G. Pandey, O. Reiser, Org. Lett. 2012, 14, 672-675
Web edition: http://dx.doi.org/10.1021/ol202857t
The photoredox-catalyzed coupling of N-aryltetrahydroisoquinoline and Michael acceptors was achieved using Ru(bpy)3Cl2 or [Ir(ppy)2- (dtb-bpy)]PF6 in combination with irradiation at 455 nm generated by a blue LED, demonstrating the trapping of visible light generated α-amino radicals. While intermolecular reactions lead to products formed by a conjugate addition, in intramolecular variants further dehydrogenation occurs, leading directly to 5,6-dihydroindolo[2,1-a]tetrahydroisoquinolines, which are relevant as potential immunosuppressive agents.
A Recyclable TEMPO catalyst for the aerobic oxidation of sulfides to sulfoxides
C. Chinusammy, O. Reiser, ChemSusChem 2010, 3, 1040-1042
Web edition: http://dx.doi.org/10.1002/cssc.201000105
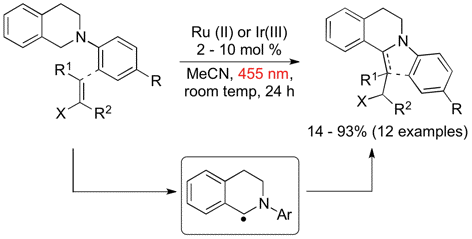
The aerobic oxidation of sulfides was accomplished with modified TEMPO A that can be readily recovered and reused by simple filtration.
The importance of 1:1 and 1:2 metal-ligand species in chiral copper(II)-bis(oxazoline) complexes for catalytic activity
M. Hager, S. Wittmann, A. Schätz, F. Pein, P. Kreitmer, O. Reiser, Tetrahedron:Asymmetry 2010, 21, 1194-1198
Web edition: http://dx.doi.org/10.1016/j.tetasy.2010.03.030
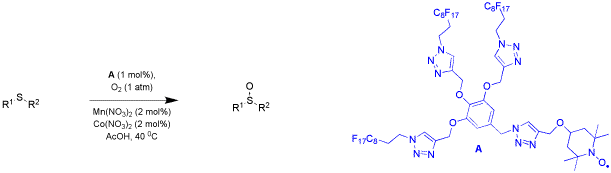
The rate ratio of different copper(II)-bis(oxazoline) complexes for catalytic activity was assessed in a simple competitive experiment. The cumulated product enantioselectivity derived from a mixture of catalysts with antipodal chiral induction provided sufficient information to determine the relative rates in asymmetric Friedel-Crafts alkylations of indoles with benzylidene malonates, moreover, the discovery of non-linear effects in the reaction system investigated gave insights into the active and resting states of the catalysts. This approach can serve as a tool to screen the activities of different catalysts when detailed kinetic measurements are not suggestive.
Iron (II)-Bis(isonitrile) Complexes: Novel Catalysts in Asymmetric Transfer Hydrogeantions of Aromatic and Heteroaromatic Ketones
A. Naik, T. Maji, O. Reiser, Chem. Commun. 2010, 46, 4475-4477
Web edition: http://dx.doi.org/10.1039/C0CC00508H
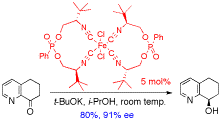
Chiral iron(II)-bis(isonitrile) complexes catalyse the transfer hydrogenation of aromatic ketones with enantioselectivities up to 91% ee, most likely via hydride transfer through imine intermediates, generated by in situ reduction of the isonitrile ligands, whereas iron acts as a Lewis acid to activate the ketone.
A Recyclable Nanoparticle-Supported Palladium Catalyst for the Hydroxycarbonylation of Aryl Halides in Water.
S. Wittmann, A. Schätz, R. N. Grass, W. J. Stark, O. Reiser, Angew. Chem. 2010, 122, 1911-1914; Angew. Chem. Int. Ed. 2010, 49, 1867-1870
Web edition: http://dx.doi.org/10.1002/anie.200906166
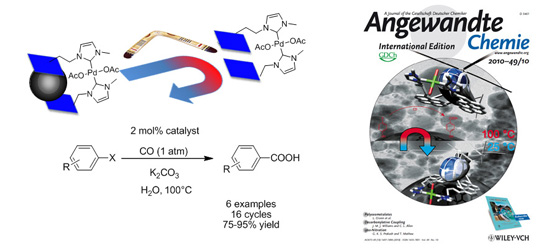
A catch-release system was established via the non-covalent attachment of a Pd(NHC)-complex on graphene-coated Co-nanoparticles. The immobilization through pyrene-tags was reversible at elevated temperatures, thus affording the homogeneous catalyst. The hydroxycarbonylation of aryl halides in water was performed with this highly active ‘boomerang’ catalyst in 16 iterative reactions. After each run, the complex was efficiently recycled, taking advantage of the extremely high magnetization of the nanosized support.
Cu(II)-Azabis(oxazoline) Complexes Immobilized on Magnetic Co/C Nanoparticles: Kinetic Resolution of 1,2-Diphenylethane-1,2-diol under Batch and Continuous-Flow Conditions.
A. Schätz, R. N. Grass, Q. Kainz, W. J. Stark, O. Reiser, Chem. Mater. 2010, 22, 305-310
Web edition: http://dx.doi.org/10.1021/cm9019099
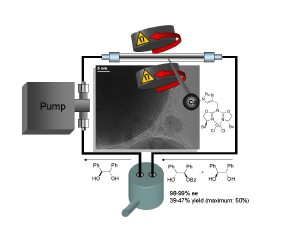
Carbon coated cobalt nanoparticles were tagged with azabis(oxazoline)-copper(II)-complexes utilizing a copper(I) catalyzed azide/alkyne cycloaddition (CuAAC) reaction and the efficacy of the resulting nanomagnetic catalyst was tested in the kinetic resolution of racemic 1,2-diols via asymmetric monobenzoylation. The novel semi-heterogeneous catalyst was examined under batch conditions and in a continuous flow-type reactor. The extremely high ferromagnetism of the cobalt cores did not only facilitate the recycling of the nanobeads via magnetic decantation in the batch reactions, but enabled also a novel continuous flow-reactor design, which allowed the efficient agitation and containment of the particles without calling for sophisticated separation strategies such as nanofiltration.
Cu(II)-Azabis(oxazoline)-Complexes Immobilized on Superparamagnetic Magnetite@Silica-Nanoparticles: A Highly Selective and Recyclable Catalyst for the Kinetic Resolution of 1,2-Diols
A. Schätz, M. Hager, O. Reiser, Adv. Funct. Mater. 2009, 19, 2109-2115
Web edition: http://dx.doi.org/10.1002/adfm.200801861
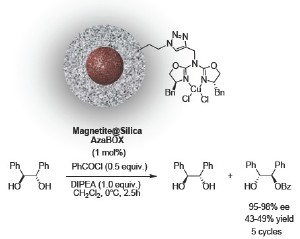
Copper(II)-azabis(oxazolines) were grafted on magnetite@silica nanoparticles via click-chemistry resulting in a highly selective heterogeneous nanocomposite that can be efficiently recycled through magnetic decantation. The catalysts were used for up to 5 iterative runs in the asymmetric benzoylation of 1,2-diols with consistent high activity or enantioselectivity.
Cyclohexane-1,2-diamines: efficient catalysts for the enantioselective conjugate addition of ketones to nitro olefins.
R. Rasappan, O. Reiser, Eur. J. Org. Chem. 2009, 1305-1308
Web edition: http://dx.doi.org/10.1002/ejoc.200801263
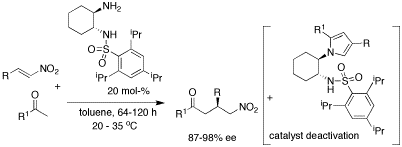
Simple monosulfonated 1,2-cyclohexyldiamines are highly enantioselective organocatalysts for the conjugate addition of ketones to nitroolefins. Focusing on aromatic ketones as the most challenging substrates, selectivities of up to 98% ee can be achieved for the title reaction. Moreover, a three-component process between the primary amine catalyst, the nitroalkene and the ketone resulting in the irreversible formation of pyrrols has been recognized as a reaction pathway for catalyst deactivation.
Immobilization of Cobalt(II) Schiff Base Complexes on Polystyrene Resin and a Study of Their Catalytic Activity for the Aerobic Oxidation of Alcohols
S. Jain, O. Reiser, ChemSusChem 2008, 1, 534-541
Web edition: http://dx.doi.org/10.1002/cssc.200800025
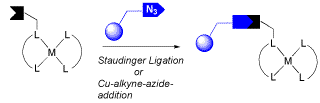
The copper catalyzed [3+2] azide-alkyne cycloaddition (CuAAC) and the Staudinger ligation were found to be readily applicable and highly efficient for the immobilization of cobalt Schiff base complexes onto polystyrene resins. Stepwise synthesis of polymer bound Schiff bases followed by their subsequent complexation with metal ions, but also direct covalent attachment of preformed homogeneous cobalt Schiff base complexes to the resins, requiring modifications on the ligand periphery of the complex without affecting its coordinated metal center, have been successfully carried out. The catalytic potential of the so prepared Polystyrene-bound cobalt Schiff bases and its comparison with their homogeneous analogues was studied for the oxidation of alcohols to carbonyl compounds using molecular oxygen as oxidant. The immobilized complexes were found to be highly efficient and even more reactive than the corresponding homogenous analogues, thus affording better yields of oxidized products within shorter reaction times. The polystyrene-bound cobalt Schiff bases could easily be recovered from the reaction mixture by simple filtration and reused as such for subsequent experiments with consistent catalytic activity.
Short α/β-Peptides as Catalysts for Intra- and Intermolecular Aldol Reactions
V. D'Elia, H. Zwicknagl, O. Reiser, J. Org. Chem. 2008, 73, 3262-3265
Web edition: http://dx.doi.org/10.1021/jo800168h
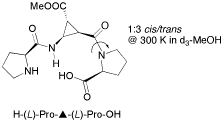
Short α/β-peptides, containing conformationally restricted cis-β-aminocyclopropylcarboxylic acid units as turn inducing elements, have been found to be efficient catalysts for inter- and intramolecular aldol reactions. The tripeptide H-(L)-Pro–▲–(L)-Pro-OH was identified to perform especially well in homogeneous and heterogeneous aqueous solution as well as in organic solvents.
C1-Symmetric Versus C2-Symmetric Ligands in Enantioselective Copper- Bis(oxazoline)-Catalyzed Cyclopropanation Reactions
J. M Fraile, J. I. Garcia, A. Gissibl, J. A. Mayoral, E. Pires, O. Reiser, M. Roldan, I. Villalba, Chem. Eur. J. 2007, 13, 8830-8839
Web edition: http://dx.doi.org/10.1002/chem.200700681
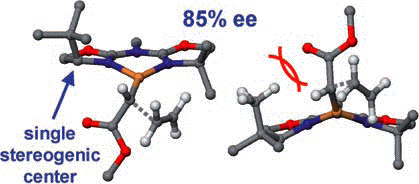
A thorough experimental and theoretical study of the enantioselective cyclopropanation of alkenes catalyzed by chiral bis(oxazoline)– and azabis(oxazoline)–copper complexes, which comprise a new family of ligands that lack C2 symmetry, has been conducted. Surprisingly high enantioselectivities were observed with some of these ligands, which were rationalized on the basis of molecular modeling studies. The course of the asymmetric induction in connection with ligand symmetry and the implications for supported enantioselective catalysts are discussed.
Synthesis of Polymer Bound Azabis(oxazoline) Ligands and their Application in Asymmetric Cyclopropanations
H. Werner, C. I. Herrerías, M. Glos, A. Gissibl, J. M. Fraile, I. Pérez, J. A. Mayoral, O. Reiser, Adv. Synth. Catal. 2006, 348, 125-132
Web edition: http://dx.doi.org/10.1002/adsc.200505197
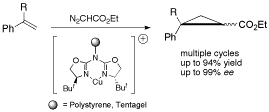
Aza(bisoxazoline) ligands were attached to various polymeric supports and the resulting immobilized ligands were evaluated in copper(I)-catalyzed asymmetric cyclopropanations. The efficiency of these transformations depends greatly on the polymeric support, on the protocol being applied for the immobilization of the ligands, and on the preparation of the catalysts.
Cu(II)-Aza(bisoxazoline)-Catalyzed Asymmetric Benzoylations
A. Gissibl, M. G. Finn, O. Reiser, Org. Lett. 2005, 7, 2325-2328
Web edition: http://dx.doi.org/10.1021/ol0505252
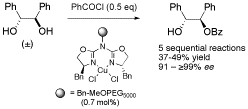
Racemic 1,2-diols and a-hydroxy carbonyl compounds can be asymmetrically benzoylated in a kinetic resolution in the presence of various Cu(II)-azabis(oxazoline) catalysts. A novel bisbenzyl substituted aza(bisoxazoline) ligand proved to be especially effective when immobilized on MeOPEG5000, giving 91 – ≥99% ee in 37 – 49% yield for each of five sequential reactions.
Cobalt(II)-Azabis(oxazoline)-Catalyzed Conjugate Reduction of α,β-Unsaturated Carbonyl Compounds.
C. Geiger, P. Kreitmeier, O. Reiser, Adv. Synth. Catal. 2005, 347, 249-254
Web edition: http://dx.doi.org/10.1002/adsc.200404295
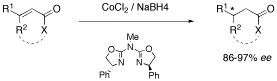
Azabis(oxazolines) prove to be superior ligands for the enantioselective, cobalt(II)-catalyzed conjugate reduction of α,β-unsaturated carbonyl compounds with sodium borohydride. β-Trisubstituted α,β-unsaturated esters and amides as well as γ-butenolides are readily converted to their corresponding saturated counterparts with enantioselectivities up to 97% ee.
The importance of complex stability for asymmetric copper-catalyzed cyclopropanations in [emim][OTf] ionic liquid: the bis(oxazoline)-azabis(oxazoline) case
J. M. Fraile, J. I. García, C. I. Herrerías, J. A. Mayoral, O. Reiser, M. Vaultier, Tetrahedron Lett. 2004, 45, 6765-6768
Web edition: http://dx.doi.org/10.1016/j.tetlet.2004.07.030
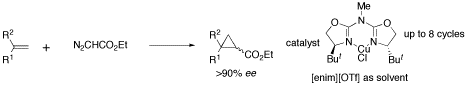
Azabis(oxazoline)–Cu complexes are more stable than their analogous based on bis(oxazoline) ligands, leading to improved recoverability (up to 8 times) when used in ionic liquid medium. The solution of the chiral catalyst can be reused even in different enantioselective cyclopropanation reactions with high enantiomeric excess (>90%).
The role of binding constant in the efficiency of chiral catalysts immobilized by electrostatic interactions. The case of azabis(oxazoline)-copper complexes
J. M. Fraile, J. I. García, C. I. Herrerías, A. Mayoral, O. Reiser, A. Socuéllamos, H. Werner, Chem. Eur. J. 2004, 10, 2997-3005
Web edition: http://dx.doi.org/10.1002/chem.200305739
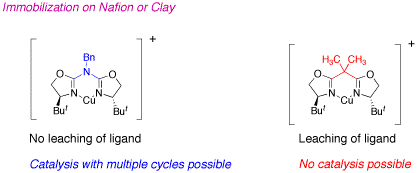
Azabis(oxazoline)-copper complexes are considerably more stable than the analogous bis(oxazoline)-copper complexes, as shown by theoretical calculations. The enhanced stability allows the efficient immobilization by means of electrostatic interactions with different anionic supports, such as clays and nafion-silica nanocomposites, without the loss of ligand observed with bis(oxazolines). In this way, enantioselectivities around 90% ee are obtained in the cyclopropanation reaction between styrene and ethyl diazoacetate with an easily recoverable solid catalyst.
Improved Synthesis of Aza-bis(oxazoline) Ligands
H. Werner, R. Vicha, A. Gissibl, O. Reiser, J. Org. Chem. 2003, 68, 10166-10168
Web edition: http://dx.doi.org/10.1021/jo0350920
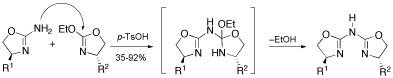
A straightforward synthesis of chiral aza-bis(oxazoline) (Azabox) ligands from commercial available aminoalcohols is described. The new protocol allows access to previously reported Azabox ligands in considerably improved yields but also to new derivatives, including non C2-symmetrical ones.
New Bis(oxazoline) Ligands with Secondary Binding Sites for the Asymmetric Cyclopropanation of Furans.
M. Schinnerl, C. Böhm, M. Seitz, O. Reiser, Tetrahedron Asymmetry 2003, 14, 765-771
Web edition: http://dx.doi.org/10.1016/S0957-4166(03)00094-6
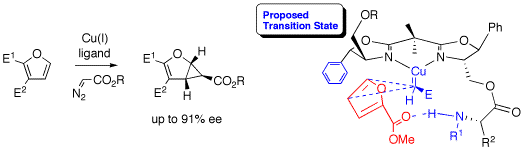
The diastereo- and enantioselective cyclopropanation of furans was achieved in up to 91% ee using a new set of chiral bis(oxazoline) ligands that are able to use secondary binding sites to enhance selectivity. In contrast, substrates such as styrene and N-Boc-pyrrole, with which no secondary interactions with the ligands can occur, gave only moderate selectivities (<50% ee).
Activation of Nitroaldol Reactions by Diethylzinc and Amino Alcohols or Diamines as Promoters.
G. Klein, S. Pandiaraju and O. Reiser, Tetrahedron Lett. 2002, 43, 7503-7506
Web edition: http://dx.doi.org/10.1016/S0040-4039(02)01768-9
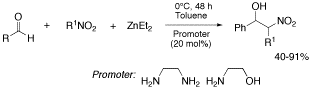
Henry reactions of nitroalkanes can be initiated with diethylzinc in the presence of catalytic amounts of 1,2-aminoethanol or 1,2-diaminoethane. In contrast, in the presence of alkylated diamines as promoters, diethylzinc addition to the aldehydes is interfering with the nitroaldol reaction.
Applications of Bis(oxazoline) Ligands in Catalysis: Asymmetric 1,2- and 1,4-Addition of ZnR2 to Carbonyl Compounds.
M. Schinnerl, M. Seitz, A. Kaiser, O. Reiser, Org. Lett. 2001, 3, 4259-4262
Web edition: http://dx.doi.org/10.1021/ol016925g
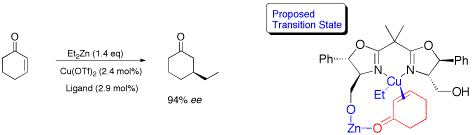
The enantioselective addition of ZnR2 to aldehydes (1,2) and cyclic enones (1,4) was accomplished using bis(oxazolines) as chiral ligands. The requirement for hydroxymethylene side chains in the ligands strongly suggests that bimetallic catalysts are decisive for high enantiocontrol in these additions.
Aza-bis(oxazolines) - New Chiral Ligands for Asymmetric Catalysis
M. Glos, O. Reiser, Org. Lett. 2000, 2, 2045-2048
Web edition: http://dx.doi.org/10.1021/ol005947k
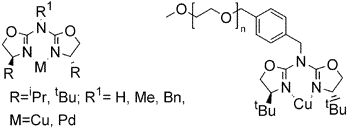
Aza-bis(oxazolines) are introduced as chiral ligands for asymmetric catalysis combining the advantages of easy availability of bis(oxazolines) and backbone variability of aza-semicorrins. Especially, the title ligands could be attached to a polymeric support, which allowed the development of easily recoverable copper(I)-catalysts for asymmetric cyclopropanation reactions.