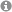UR Home
UR Home
Reviews
Nanoparticles as Semi-Heterogeneous Catalyst Supports
A. Schätz, O. Reiser, W. J. Stark, Chem. Eur. J. 2010, 16, 8950-8967
Web edition: http://dx.doi.org/10.1002/chem.200903462
Nanoparticles can serve as semiheterogeneous supports since they readily disperse in common solvents and combine high surface area with excellent accessibility. Reversible agglomeration through solvent changes and magnetic separation provide technically attractive alternatives to classical catalyst filtration.
Synthesis of biological active guaianolides with a trans annulated lactone moiety
A. Schall,
O. Reiser, Eur. J. Org. Chem. 2008, 2353-2364
Web edition: http://dx.doi.org/10.1002/ejoc.200700880
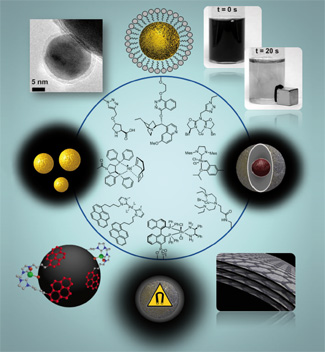
The biosynthetic pathways of sesquiterpene lactones are described in conjunction with recent developments in the total syntheses of various biologically active guaianolides highlighting different strategies towards the 5,7,5-membered ring system. Oxidative diol-cleavages, aldol-reactions and intra-molecular cyclopropanations were used as key reactions to construct the racemic guaianolide core system. Radical cyclizations as well as ring closing metathesis were successfully applied in asymmetric approaches. Biomimetic reactions were also applied as versatile tools for the construction of these highly complex natural products.
Metal(bisoxazoline) complexes: From coordination chemistry to asymmetric catalysis
R. Rasappan, D. Laventine, O. Reiser, Coord. Chem. Rev. 2008, 252, 702-714
Web edition: http://dx.doi.org/10.1016/j.ccr.2007.11.007
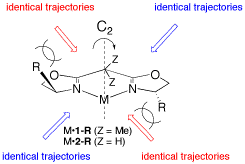
Metal-bis(oxazoline) complexes have proven to be one of the most versatile classes of chiral catalysts being able to promote a large variety of organic transformations. The coordination geometry and electronic properties play an integral role for the asymmetric induction exhibited by these complexes and, aided by X-ray structure analysis and NMR studies, rules have been recognized to understand their function. Based on the most common complex geometries observed, i.e. tri, tetra- (tetrahedral or square-planar), penta- (trigonal-bipyramidal or square-pyramidal), or hexacoordination (octahedral) a mechanistic picture has emerged that allows control of enantioselectivity in reactions in a highly predictable and tunable way, demonstrating the power of combining the knowledge of coordination chemistry and asymmetric catalysis.
Synthetic Approaches towards structurally divers γ-Butyrolactone Natural Product-like Compounds
M. Seitz, O. Reiser, Curr. Opin. Chem. Biol. 2005, 9, 285-292
Web edition: http://www.dx.doi.org/10.1016/j.cbpa.2005.03.005
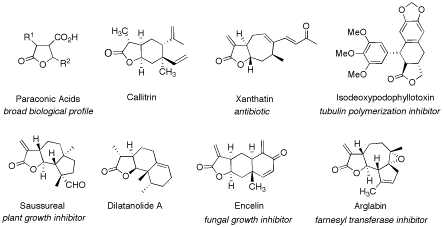
Recent strategies for diversity-oriented synthetic approaches towards γ-butyrolactone natural product-like compounds with a special focus on catalytic asymmetric methodology are discussed.
Total Synthesis of Stemoamide using ring-closing-metathesis - a comparison
E. Jezek, O. Reiser, Chemtracts - Organic Chemistry 2005, 18, 200-206
Web edition: http://www.datatrace.com/online-journals/emailurl.html?618627
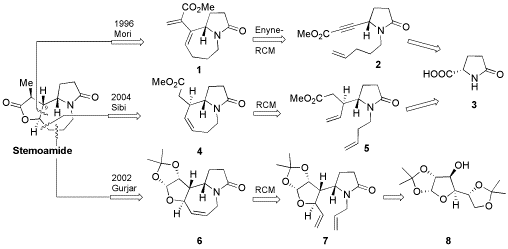
Synthetic strategies to stemoamide using ring closing metathesis as a key step are reviewed.
Molekulares Spiegelkabinett
R. Demuth, O. Reiser, Spektrum der Wissenschaft 2005, Heft 2, 70-73
Web edition: http://www.wissenschaft-online.de/artikel/771160

Auch im Reich der Moleküle kann es wichtig sein, zwischen links und rechts zu unterscheiden.
Paraconic Acids: The Natural Products from Lichen Symbiont
R. Bandichhor, B. Nosse, O. Reiser, Top. Curr. Chem. 2005, 243, 43-72
Web edition: http://dx.doi.org/10.1007/b96881
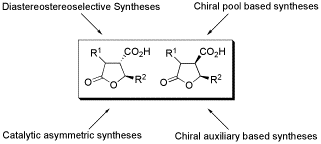
Synthetic approaches towards paraconic acids are reviewed.
Tandem epoxidation/stereoselective intramolecular [4+3] cycloaddition reaction
A. Schall, O. Reiser, Chemtracts - Organic Chemistry 2004, 17, 436-441
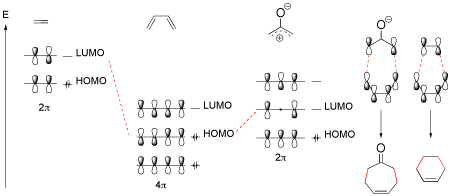
The scope and limitations of [4+3] cycloaddition reactions involving chiral oxyallyl cations derived from allenamides is discussed.
Cyclopropanation with Fischer Acyloxycarbene Complexes: Preparation of Cyclopropane and Cycloheptane-Fused g-Lactones
R.B. Chhor, O. Reiser, Chemtracts Organic Chemistry 2004, 17, 67-71
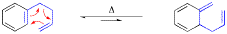
The scope and limitation of the aromatic Cope rearrangement and its application in synthesis is reviewed.
Synthesis and Application of b-Aminocyclopropanecarboxylic Acids.
F. Gnad, O. Reiser, Chem. Rev. 2003, 103, 1603-1624
Web edition: http://dx.doi.org/10.1021/cr010015v
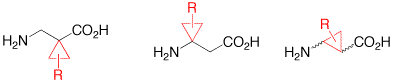
The synthesis and application of cyclopropyl-modified β-alanines is reviewed.
The Sharpless Asymmetric Aminohydroxylation - Scope and Limitation.
D. Nilov, O. Reiser, Adv. Synth. Catal. 2002, 344, 1169-1173
Web edition: http://dx.doi.org/10.1002/1615-4169(200212)344:10<1169::AID-ADSC1169>3.0.CO;2-G
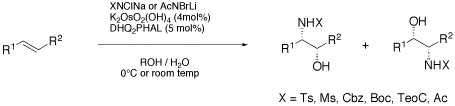
The asymmetric aminohydroxylation (AA) has emerged as a valuable tool in organic synthesis. Recent developments, such as ligandless variants, new nitrogen reagents and new substrates have considerably broadened the utility of the process. Nevertheless, the understanding of the AA, both in terms of mechanism as well as applicability to common synthetic tasks is still limited. This article summarizes the scope and limitation of the AA with special emphasis on recent advances.
Polymer bound bis(oxazolines) for asymmetric catalysis.
O. Reiser, Chimica Oggi/Chemistry Today 2002, 73-77
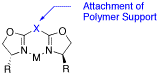
Strategies for covalent immobilization of bis(oxazoline) ligands are reviewed.
The Photochemistry of 2-(1-Naphthyl)ethyl Benzoates: Cycloaddition and Intramolecular Exciplex Formation.
R. Bandichhor, O. Reiser, Chemtracts - Organic Chemistry 2001, 14, 773-777
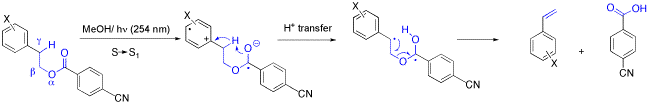
Norrish-II-fragmentations induced by intramolecular electron transfer are reviewed.
Palladium-katalysierte Kohlenstoff-Kohlenstoff-Kupplungsreaktionen.
O. Reiser, Chemie in unserer Zeit 2001, 35, 94-100
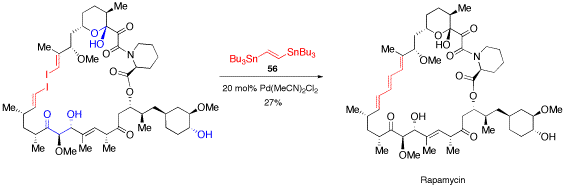
Palladium-catalyzed cross coupling reactions are reviewed.
A Demonstration of the Primary Stereoelectronic Effect in the Baeyer-Villiger Oxidation of α-Fluorocyclohexanones.
O. Reiser, Chemtracts Organic Chemistry 2001, 14, 94-99
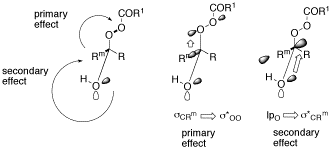
Stereoelectronic effects in the Baeyer-Villager oxidation are reviewed.
Katalysatoren: Schwerarbeiter in Chemie und Biologie.
P. Kreitmeier, O. Reiser, Blick in die Wissenschaft 2000, 12, 14-19
Was sind Katalysatoren und wie funktionieren sie?
Around and Beyond Cram
A. Mengel, O. Reiser, Chem. Rev. 1999, 99, 1191-1223
Web edition: http://dx.doi.org/10.1021/cr980379w
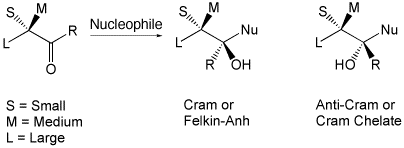
The different models for 1,2- and 1,3-induction of nucleophile and electrophile additions to carbonyl compounds and alkenes are reviewed.