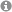UR Home
UR Home
Publications
31. Trendbericht Photochemie 2025
30. „Asymmetric Migratory Tsuji-Wacker Oxidation Enables the Enantioselective Synthesis of Hetero- and Isosteric Diarylmethanes“
E. Frank,† S. Park,† E. Harrer, J. L. Flügel, M. Fischer, P. Nuernberger, J. Rehbein,* A. Breder,* J. Am. Chem. Soc. 2024 146 (50), 34383-34393 († these authors contributed equally) https://pubs.acs.org/doi/10.1021/jacs.4c09405
28. "Intermolecular Aza-Wacker Coupling of Alkenes with Azoles by Photo-Aerobic Selenium-π-Acid Multicatalysis"
T. Lei,† T. Appleson,† A. Breder,* ACS Catal. 2024, 14, 9586−9593. (†these authors contributed equally)
27. "Mechanistic Analysis Reveals Key Role of Interchalcogen Multicatalysis in Photo-Aerobic 3-Pyrroline Syntheses by Aza-Wacker Cyclizations"
S. Graf,† H. Pesch,† T. Appleson, T. Lei, A. Breder,* I. Siewert,* ChemSusChem 2024, e202301518 (manuscript accepted, †these authors contributed equally)
26. "Asymmetric Photo-Aerobic Lactonization and aza-Wacker Cyclization of Alkenes Enabled by Ternary Selenium-Sulfur Multicatalysis"
T. Lei†, S. Graf†, C. Schöll, F. Krätzschmar, B. Gregori, T. Appleson, A. Breder*, ACS Catal. 2023, 13, 16240−16248 (†these authors contributed equally) (https://pubs.acs.org/doi/10.1021/acscatal.3c04443)
25. "Synthesis of 1,3-Dioxan-2-ones by Photo-Aerobic Selenium-π-Acid Multicatalysis"
K. A. Müller, C. H. Nagel, A. Breder*, Eur. J. Org. Chem. 2023, 26, e2022011 (invited manuscript)
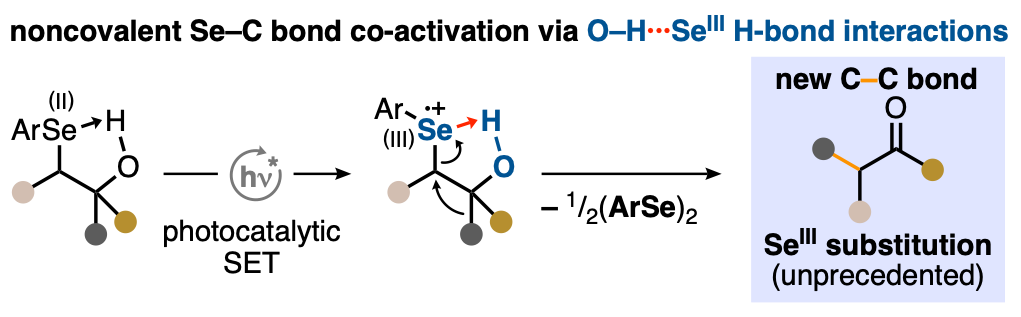
24. "Hydrogen-Bond-Modulated Nucleofugality of SeIII Species to Enable Photoredox-Catalytic Semipinacol Manifolds"
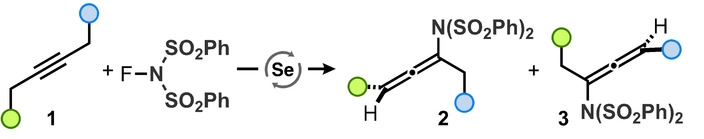
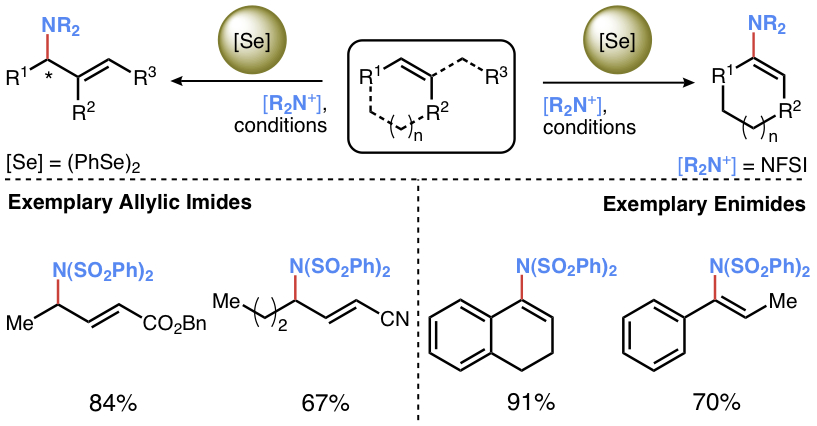
23. "Synthesis of Aminoallenes via Selenium-π-Acid-Catalyzed Cross-Coupling of N-Fluorinated sunlfonimides with Simple Alkynes"
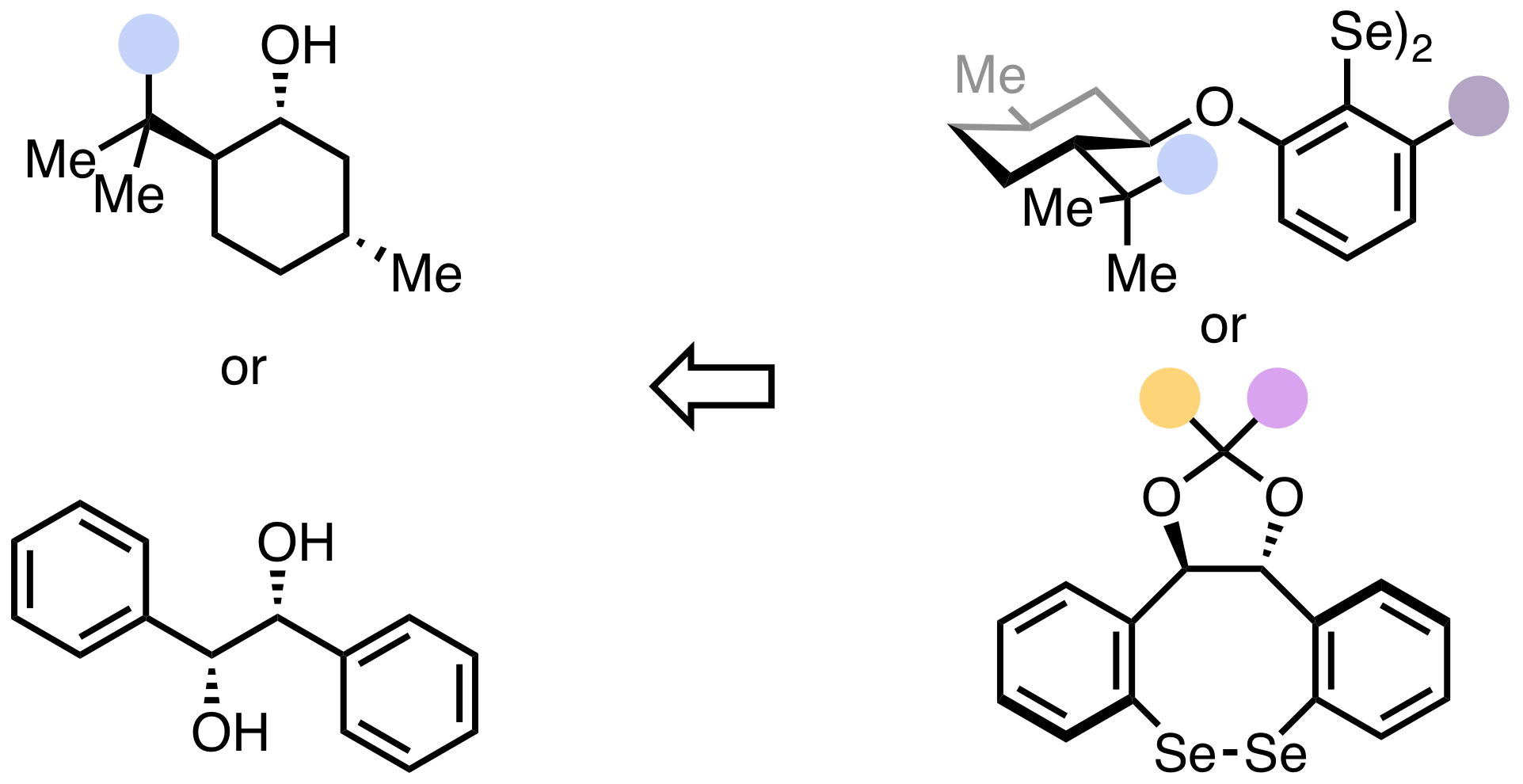
22. "Rational Design of Chiral Selenium-π-Acid Catalysts"
F. Krätzschmar, S. Ortgies, R. Y. N. Willing, A. Breder,* Catalysts 2019, 9 (2), 153.
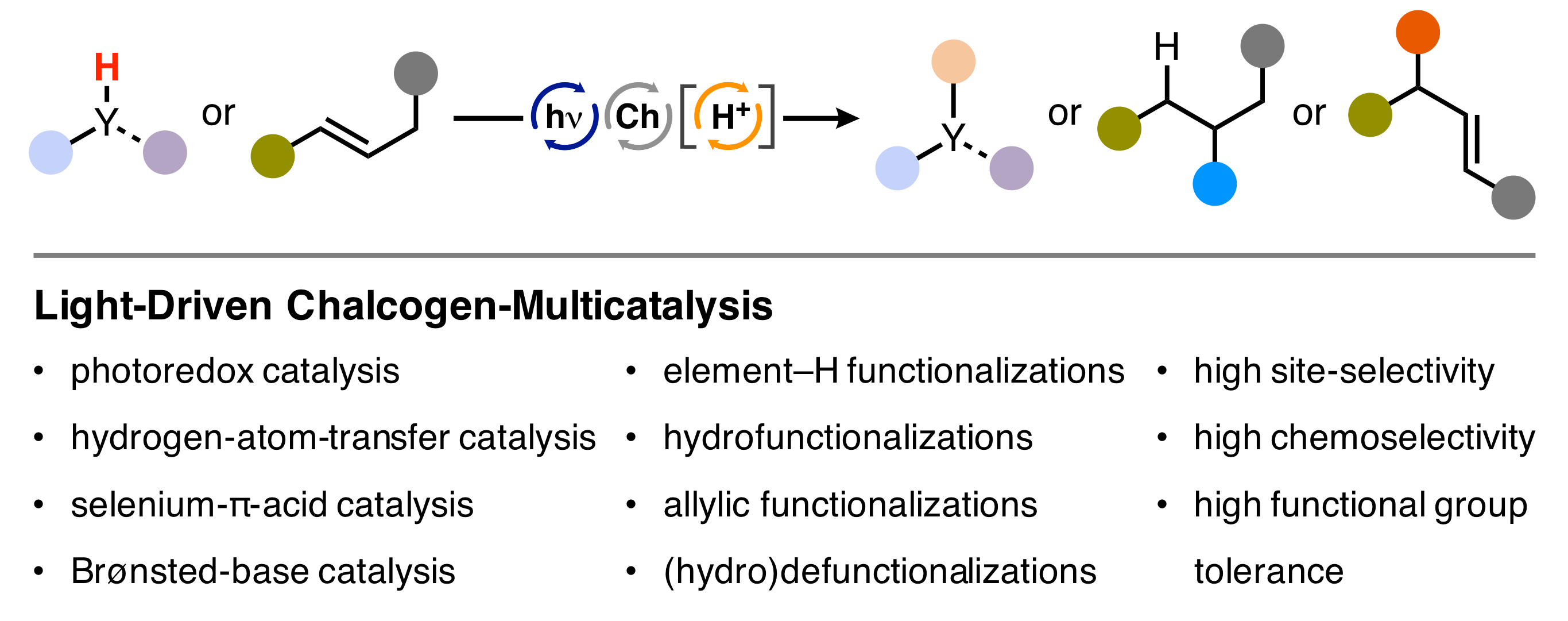
21. "Light-Driven Single-Electron-Transfer Processes as an Enabling Principle in Sulfur- and Selenium-Multicatalysis"
A. Breder,* C. Depken, Angew. Chem. Int. Ed. 2019, 58, 17130-17147.
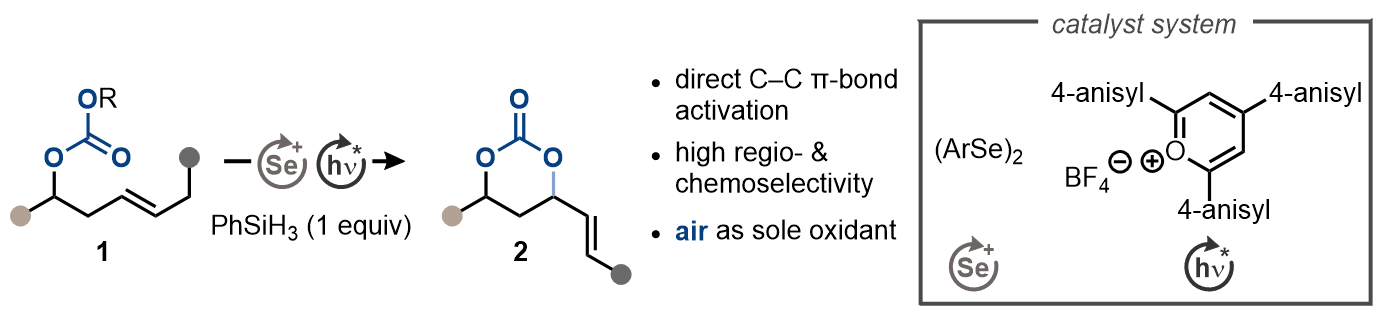
20. "Aerobic Allylation of Alcohols with Non-Activated Alkenes Enabled by Light-Driven Selenium-π-Acid Catalysis"
K. Rode, M. Palomba, S. Ortgies, R. Rieger, A. Breder,* Synthesis 2018, 50, 3875. (invited Feature Article)
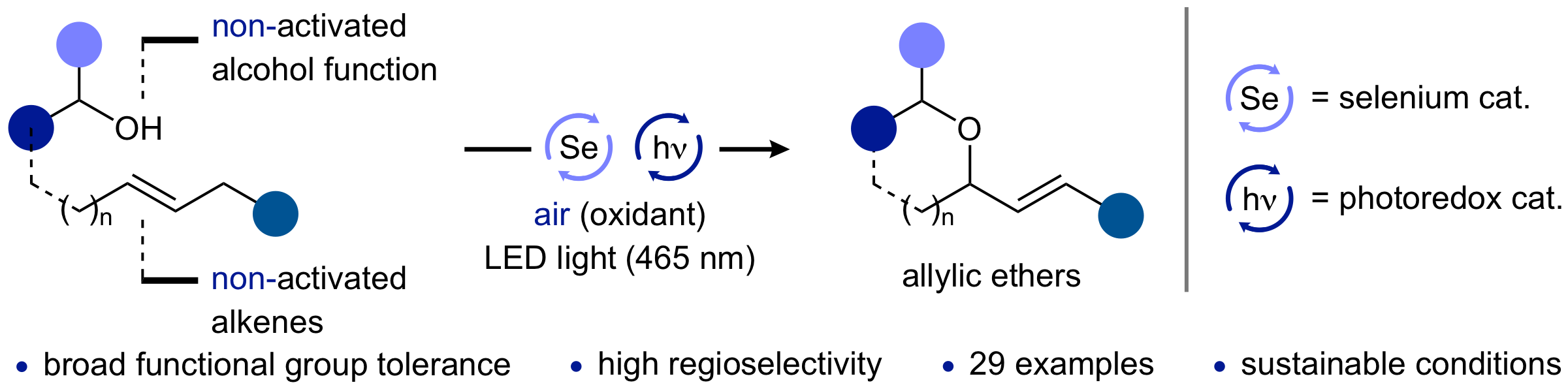
19. "Mechanistic Studies on the Anodic Functionalization of Alkenes Catalyzed by Diselenides"
M. Wilken, S. Ortgies, A. Breder,* I. Siewert,* ACS Catal. 2018, 8, 10901
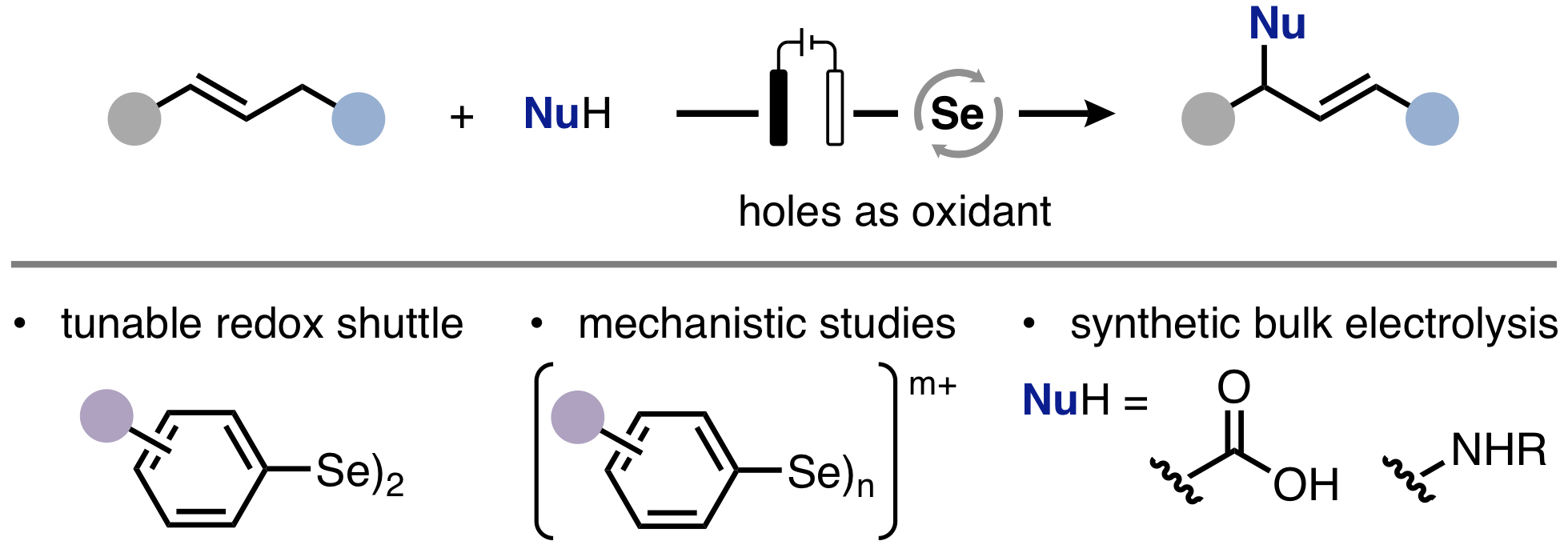
18. "Photocatalytic Aerobic Phosphatation of Alkenes"
C. Depken,° F. Krätzschmar,° R. Rieger, K. Rode, A. Breder,*
Angew. Chem. Int. Ed. 2018, 57, 2459.
Angew. Chem. 2018, 130, 2484.
(This manuscript was highlighted in Synfacts 2018, 14, 314)
(° these authors contributed equally to this manuscript)
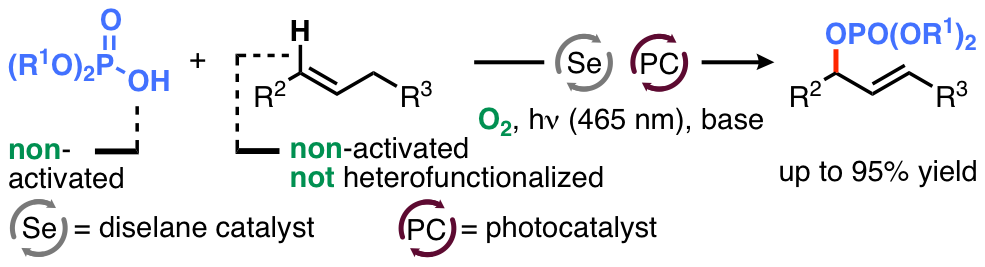
17. "Mechanistic and Synthetic Investigations on the Dual Selenium-π-Acid/Photoredox Catalysis in the Context of the Aerobic Dehydrogenative Lactonization of Alkenoic Acids"
S. Ortgies, R. Rieger, K. Rode, K. Koszinowski, J. Kind, C. M. Thiele, J. Rehbein, A. Breder,* ACS Catal. 2017, 7, 7578.
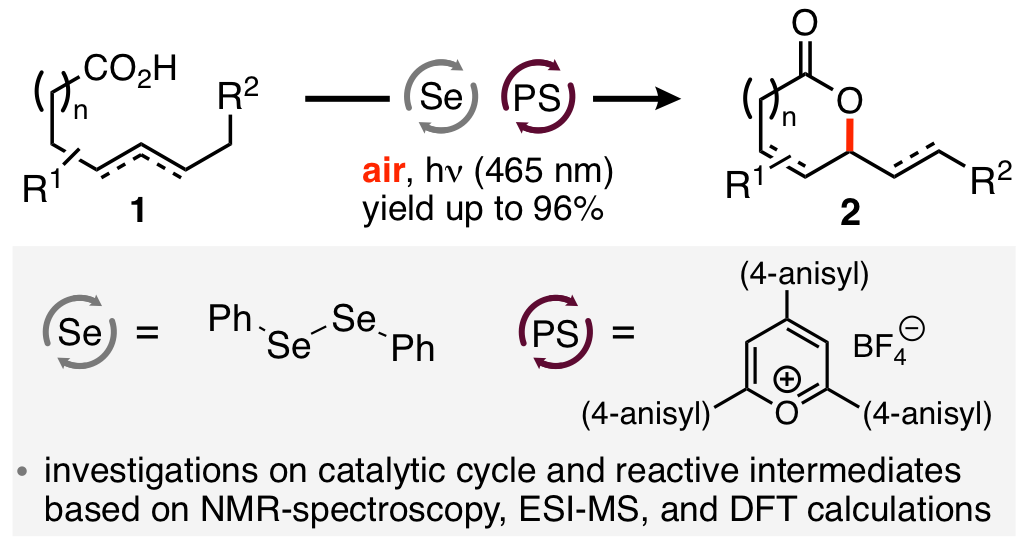
16. "Oxidative Alkene Functionalizations via Selenium-π-Acid Catalysis"
S. Ortgies, A. Breder,* ACS Catal. 2017, 7, 5828
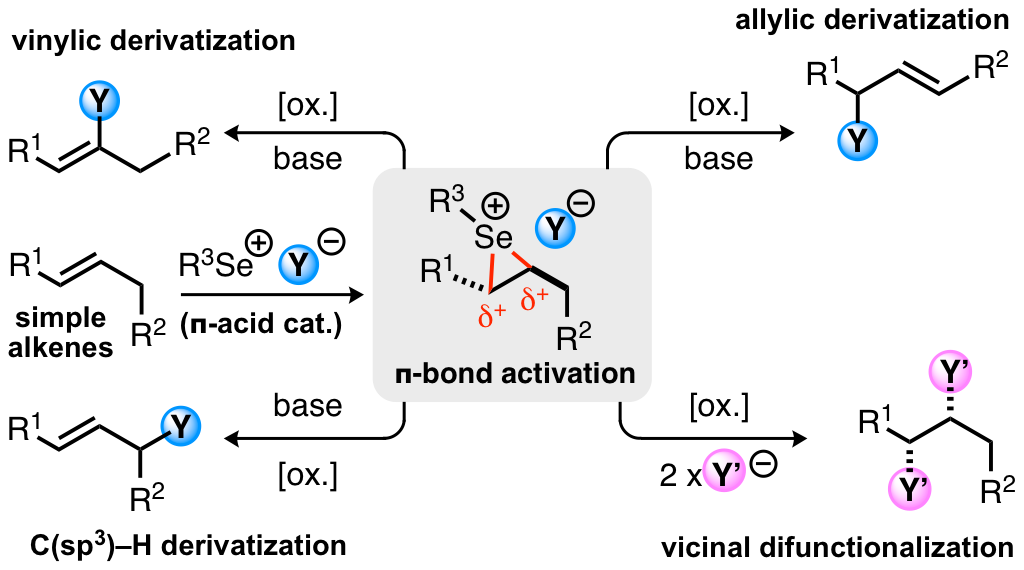
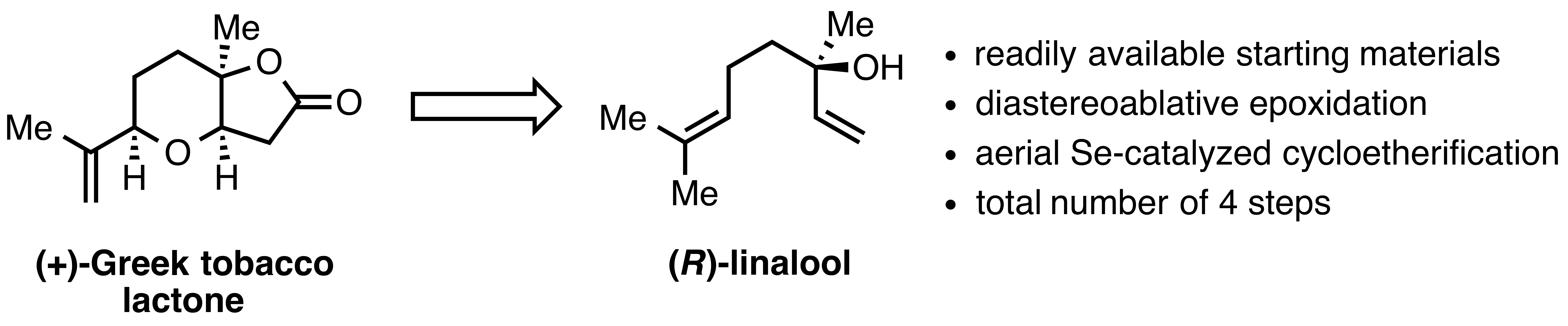
15. "Synthesis of (+)-Greek Tobacco Lactone via a Diastereoablative Epoxidation and a Selenium-Catalyzed Oxidative Cyclization"
S. Leisering, I. Riano, C. Depken, L. Gross, M. Weber, D. Lentz, R. Zimmer, C. B. W. Stark,* A. Breder,* M. Christmann,* Org. Lett. 2017, 19, 1478
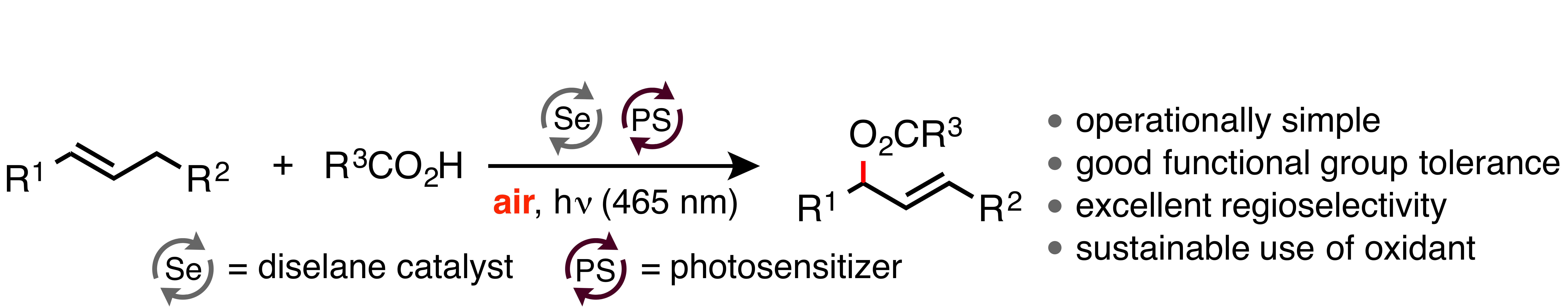
14. "Oxidative Allylic Esterification of Alkenes by Cooperative Selenium-Catalysis Using Air as the Sole Oxidant"
S. Ortgies, C. Depken, A. Breder,* Org. Lett. 2016, 18, 2856
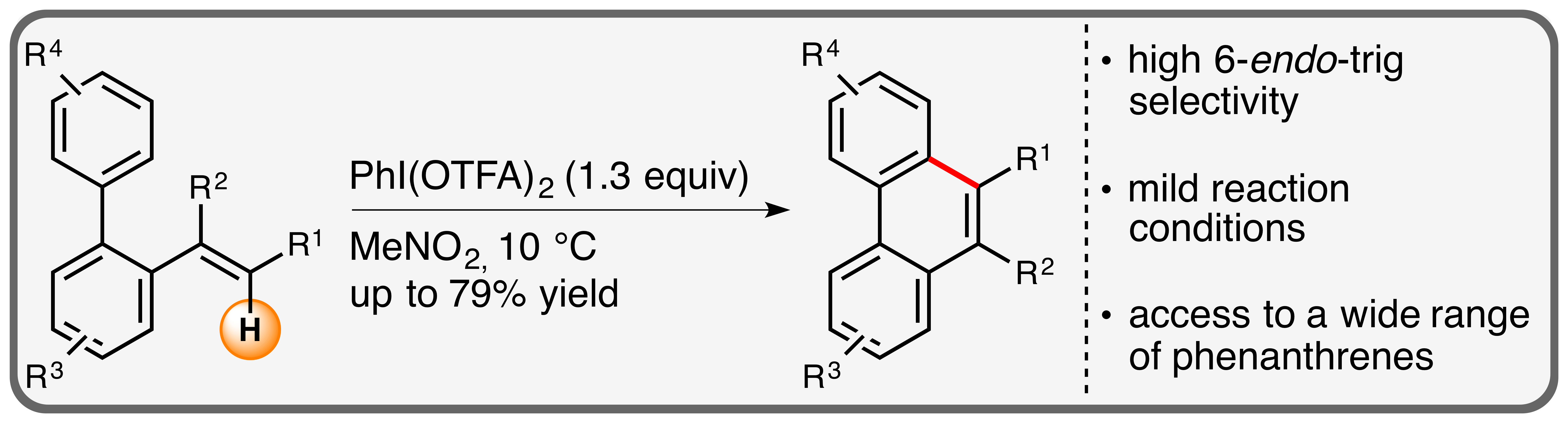
13. "Iodine(III)-mediated oxidative intramolecular arene-alkene coupling exemplified in the synthesis of phenanthrenes"
C. Depken, F. Krätzschmar, A. Breder,* Org. Chem. Front. 2016, 3, 314-318 (invited contribution to the themed collection "2015 Emerging Investigators by OCF")
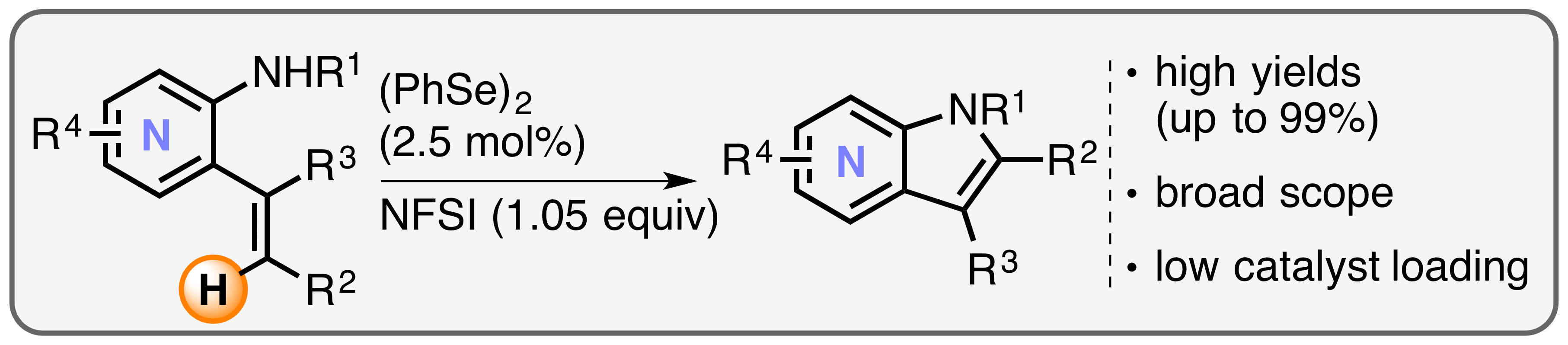
12. "Selenium-Catalyzed Oxidative C(sp2)–H Amination of Alkenes Exemplified in the Expedient Synthesis of (Aza-)Indoles"
S. Ortgies, A. Breder,* Org. Lett. 2015, 17, 2748-2751
11. "Recent developments in sulfur- and selenium-catalyzed oxidative and isohypsic functionalization reactions of alkenes"
A. Breder,* S. Ortgies, Tetrahedron Lett. 2015, 56, 2843-2852 (invited Digest Article)
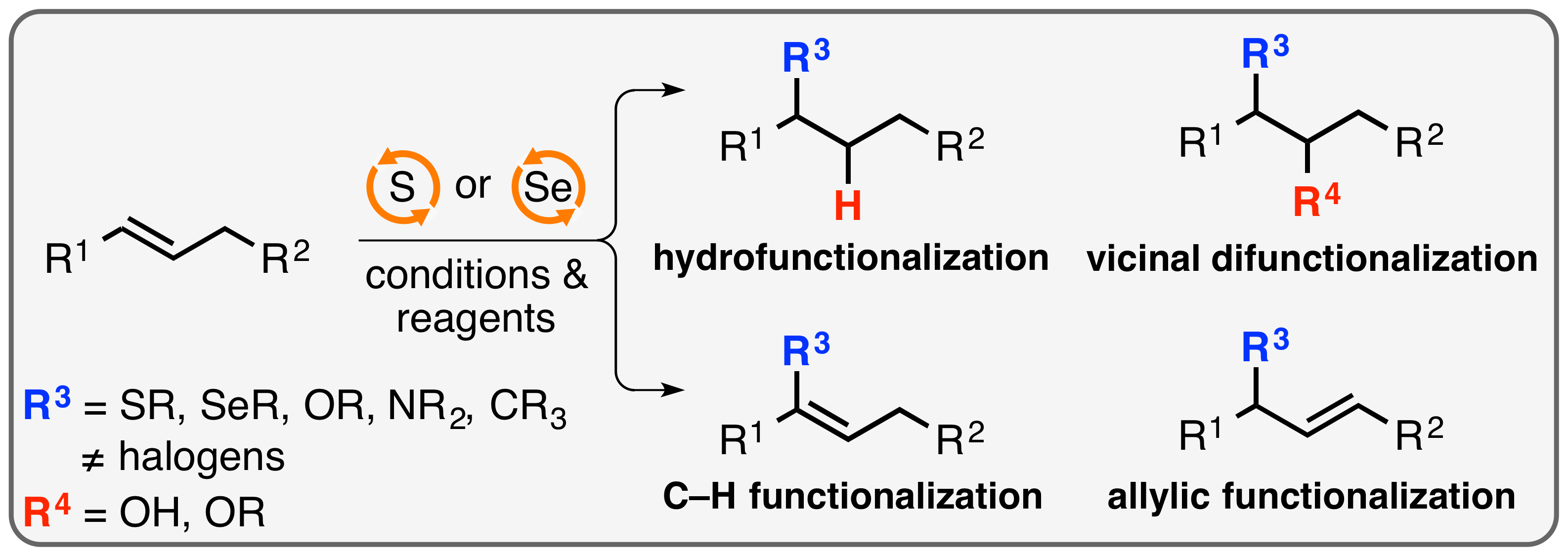
10. "Selenium-Catalyzed C(sp3)–H Acyloxylation: Application in the Expedient Synthesis of Isobenzofuranones" |Hot Paper|
F. Krätzschmar, M. Kaßel, D. Delony, A. Breder,* Chem. Eur. J. 2015, 21, 7030-7034
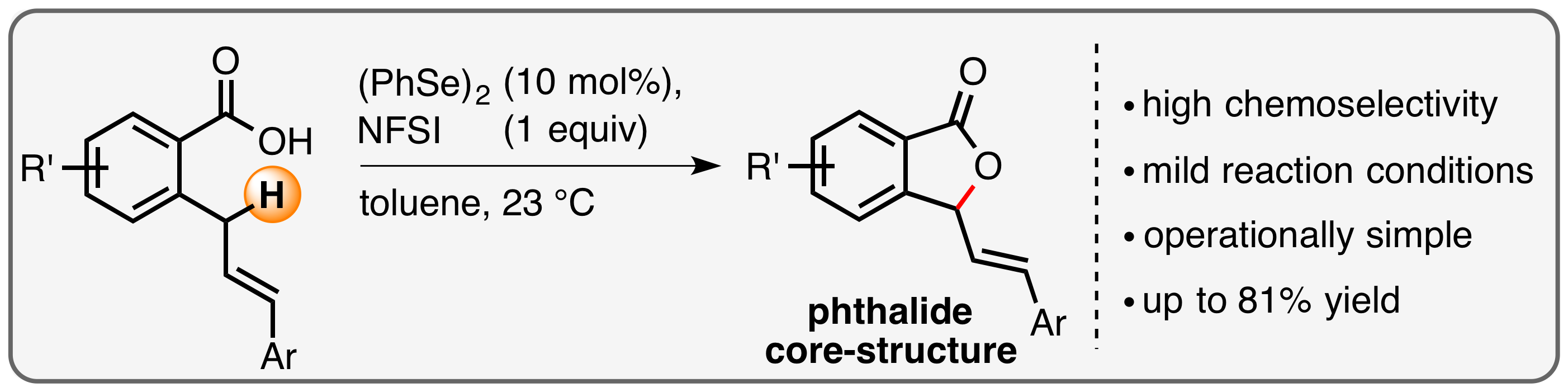
8. "Oxidative Allylic Amination Reactions of Unactivated Olefins – At the Frontiers of Palladium and Selenium Catalysis"
A. Breder,* Synlett 2014, 25, 899-904 (invited Synpacts article)
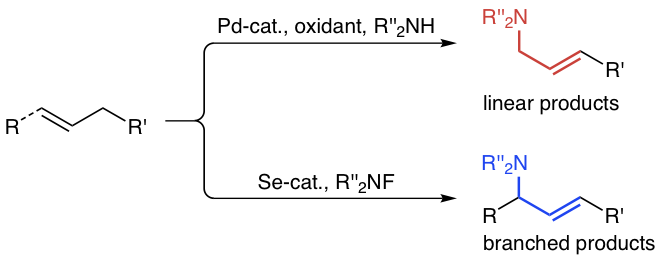
7. "Direct Oxidative Allylic and Vinylic Amination of Alkenes through Selenium Catalysis"
J. Trenner, C. Depken, T. J. Weber, A. Breder,*
Angew. Chem. Int. Ed. 2013, 52, 8952-8956.
Angew. Chem. 2013, 125, 9121-9125

2008-2012 (Ph.D & Postdoc Work)
6. "Atom-Economical Synthesis of Functionalized Cycloalkanes via Catalytic Redox Cycloisomerization"
B. M. Trost, A. Breder, K. Bao, Org. Lett. 2012, 14, 1708-1711
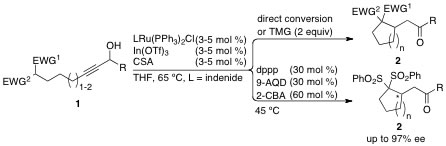
5. "Towards the Synthesis of Massadine: A Unified Strategy for the Stereoselective Synthesis of the Carbocyclic C,D-Ring Subunit"
A. Breder, G. M. Chinigo, A. W. Waltman, E. M. Carreira, Chem. Eur. J. 2011, 17, 12405-12416
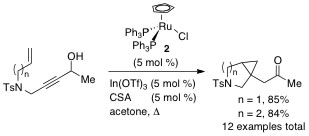
4. "Propargyl Alcohols as β-Oxocarbenoid Precursors for the Ruthenium-catalyzed Cyclopropanation of Unactivated Olefins by Redox Isomerization"
B. M. Trost, A. Breder, B. M. O'Keefe, M. Rao, A. W. Franz, J. Am. Chem. Soc. 2011, 133, 4766-4769
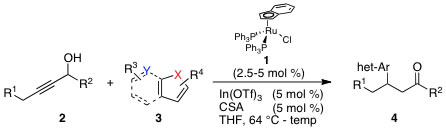
3. "An Atom-Economical Access to β-Heteroarylated Ketones from Propargylic Alcohols via Tandem Ruthenium/Indium-Catalysis"
B. M. Trost, A. Breder, Org. Lett. 2011, 13, 398-401
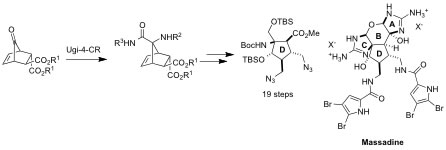
2. "Ugi-4-Component Reaction Enabling Rapid Access to the Core Fragment of Massadine"
G. M. Chinigo, A. Breder, E. M. Carreira, Org. Lett. 2011, 13, 78–81
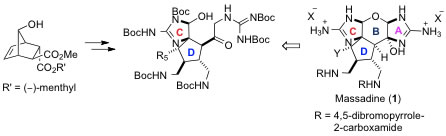
1. "Enantioselective Synthesis of the Carbocyclic D-Ring Subunit of Massadine"
A. Breder, G. M. Chinigo, A. W. Waltman, E. M. Carreira, Angew. Chem. Int. Ed. 2008, 47, 8514–8517 (highlighted in CHIMIA 2008, 62, 980)
Secretary: CH 23.1.80
Phone: +49 (0)941 943-4626
EMail:sekretariat.breder@ur.de
Phone: +49 (0)941 943-4802